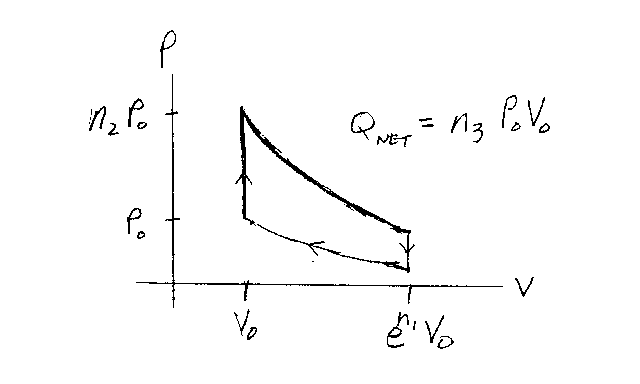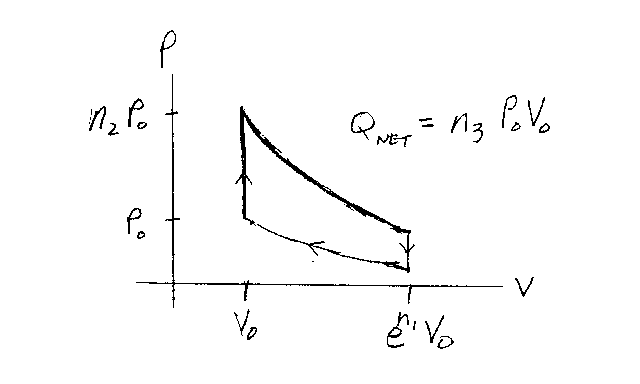Problem D10:
Consider an ideal gas that is confined to a container. The gas
undergoes the cyclic quasi-static process shown in the graph below. The cycle consists of
two isothermal processes (constant T) bounded by two isometric processes (constant V).
The gas starts off with a pressure of P0 and a volume of V0.
The gas is first heated up with a constant volume to a pressure n2P0.
Then it expands isothermally to a volume en1V0. Next it
cools down with a constant volume. Finally, it is compressed isothermally to its original
state. If the net heat energy given to the gas is Qnet=n3
P0V0, what is n3?
Note that n1, n2 and n3 are unitless.
If you are currently in my class, you can record your grade by entering your name and student ID
number (without the leading zeros) below and clicking on "record grade".