Problem D11:
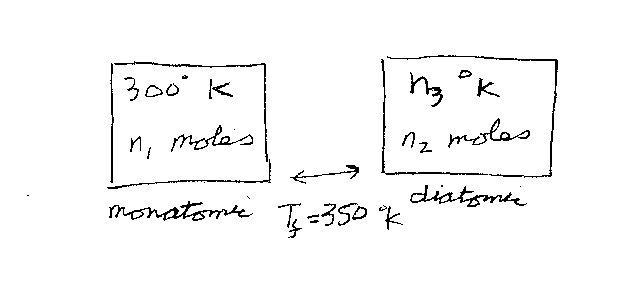
Consider two ideal gases in two different containers. One container has n1
moles of a monatomic gas that starts off at a temperature of 300 Deg K. The other
container has n2 moles of a diatomic gas that starts off at a temperature
of n3 Deg K. The two containers are brought in contact and and
come to thermal equilibrium with a final temperature of 350 Deg K.
What was the initial temperature n3 (in Deg K) of the diatomic gas?
Note that n1 and n2 are unitless. n3 is in units
of Deg K.
If you are currently in my class, you can record your grade by entering your name and student ID
number (without the leading zeros) below and clicking on "record grade".

